Research
Neutral Pyridine Ligands
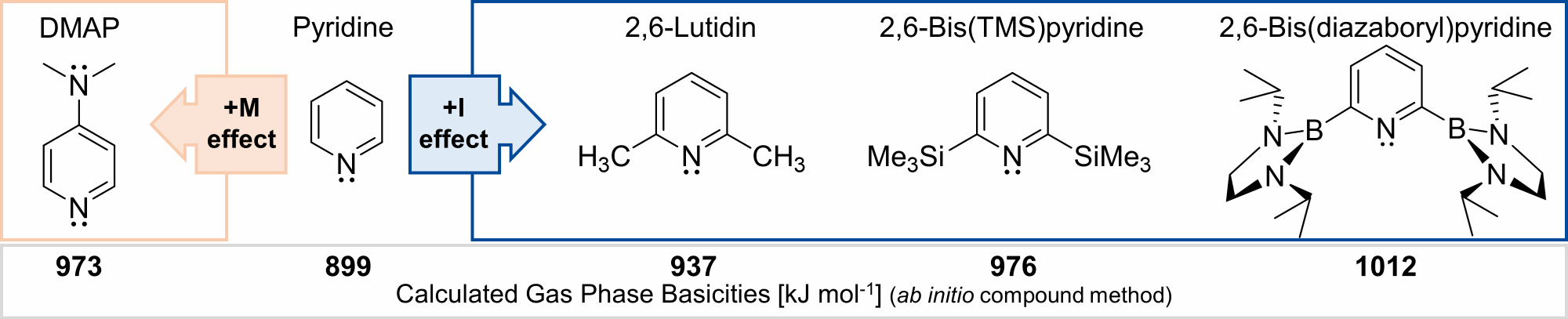
The most prominent example for a pyridine with increased basicity is DMAP. This is due to the +M-effect of the dimethylamino-group. Our group is using a different approach in making very basic and even superbasic pyridines by utilizing groups with a +I-effect. Electropositive groups at the 2,6-positions at pyridine push electron density towards the pyridine’s nitrogen atom thus increasing its basicity. While alkyl- and silyl-groups block the nitrogen atom due to their steric bulk, the even more electropositive diazaboryl-groups are planar and can orientate perpendicular to the pyridine plane, allowing for its application in coordination chemistry.
J. Schröder, D. Himmel, T. Böttcher, Chem. Eur. J. 2017, 23, 10763-10767. DOI: 10.1002/chem.201702890
Anionic Pyridine Ligands
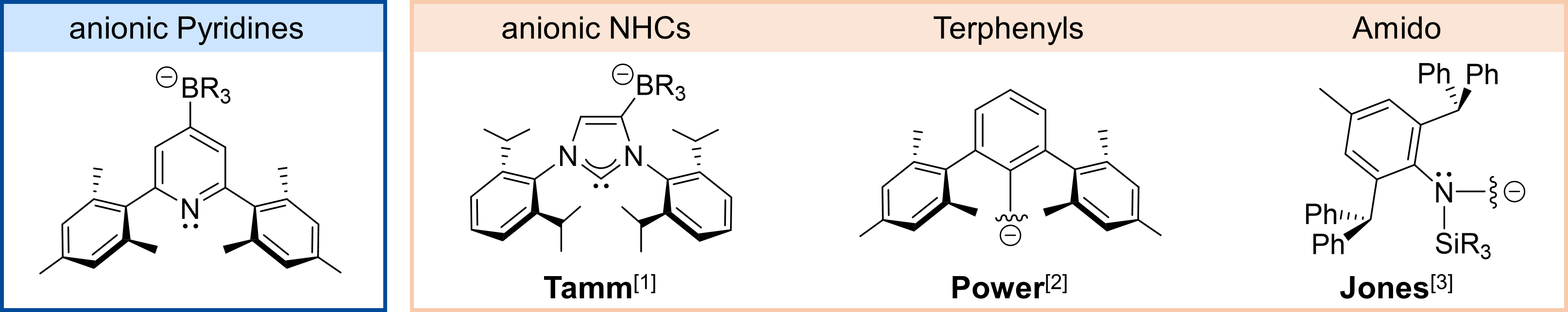
Anionic and monodentate ligands play a significant role in modern main group chemistry. There are numerous examples for very unusual compounds stabilized with anionic NHCs, terphenyls and sterically demanding amido-ligands. Our group introduced a borate function to the 4-position of 2,6-dimesitylpyridine as a first example of a monodentate anionic pyridine ligand. The introduction of the negatively charged group makes 4-(Ph3B)-2,6-Mes2Py (pKa = 18.46) become more basic than DMAP (pKa = 17.95).
N. M. C. Schmidlin, M. Lõkov, I. Leito, T. Böttcher, Chem. Eur. J. 2018, 24, 16851-16856. DOI: 10.1002/chem.201803626
[1] A. Nasr, A. Winkler, M. Tamm, Coord. Chem. Rev. 2016, 316, 68-124. [2] B. R. Barnett et al., Inorg. Synth. 2018, 37, 85-122. [3] D. L. Kays, Chem. Soc. Rev. 2016, 45, 1004-1018.
A Stable Pyridine Radical Anion
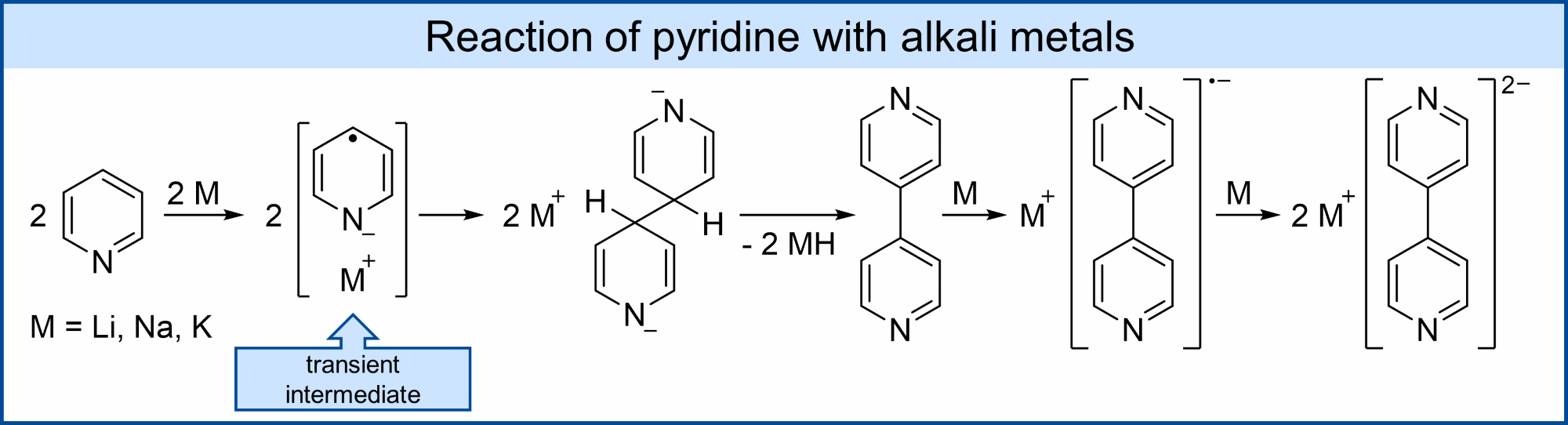
Pyridine reacts with alkali metals to eventually yield bipyridines with 4,4’-bipyridine being the favored product. The reaction proceeds via the dimerization of the transient pyridine radical anion. This radical anion has a lifetime of only seconds to minutes in solution, depending on the reaction conditions and has only been characterized by means of EPR and UV/vis spectroscopy.
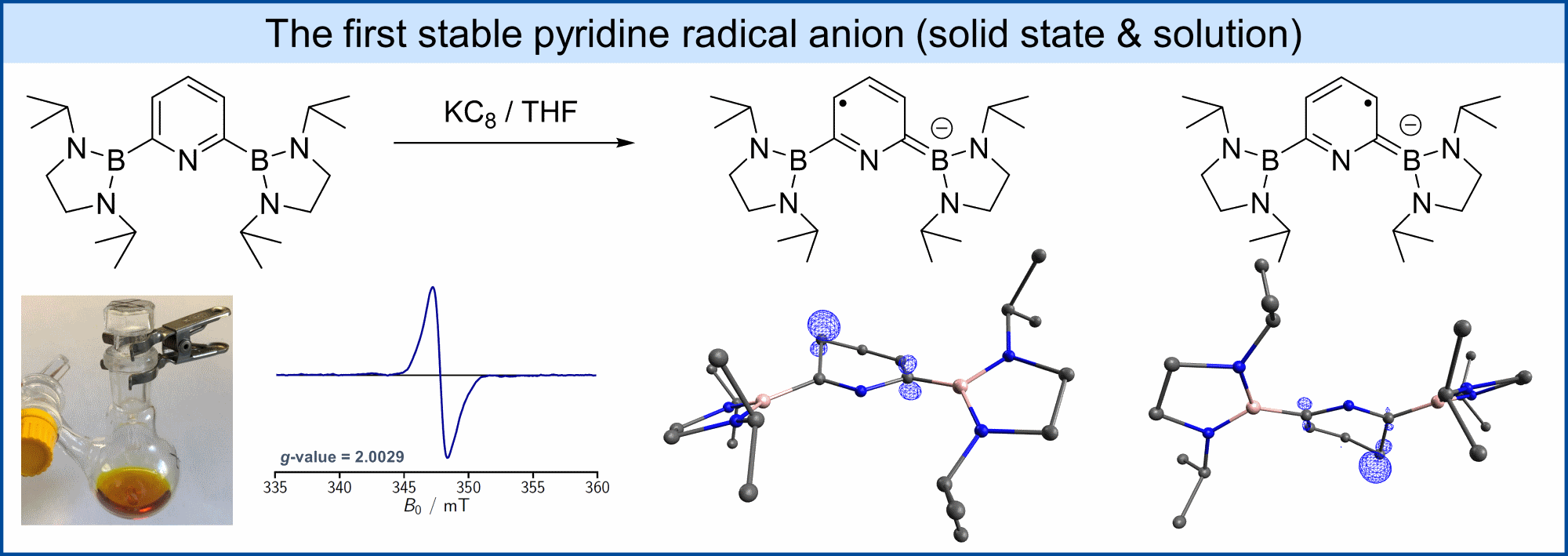
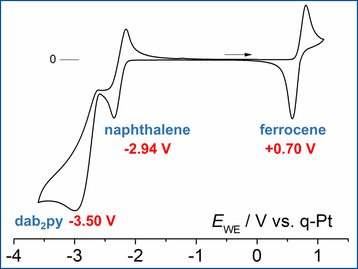
Reduction of 2,6-bis(diazaboryl)pyridine (dab2py) with KC8 gives a yellow colored solution. Crystallization of the reaction product and determination of the structure revealed two conformeric pyridine radical anions. Mulliken and NBO analysis showed that the spin density and negative charge are mainly distributed over the atoms of the pyridine ring, which makes this the first example of a pyridine radical anion that is stable as a solid and in solution.
Cyclovoltammographic measurements showed that the pyridine radical anion is more reducing than naphthalenide by more than 0.5 V.
J. Schröder, D. Himmel, D. Kratzert, V. Radtke, S. Richert, S. Weber, T. Böttcher, Chem. Commun. 2019, 55, 1322-1325. DOI: 10.1039/c8cc09700c

